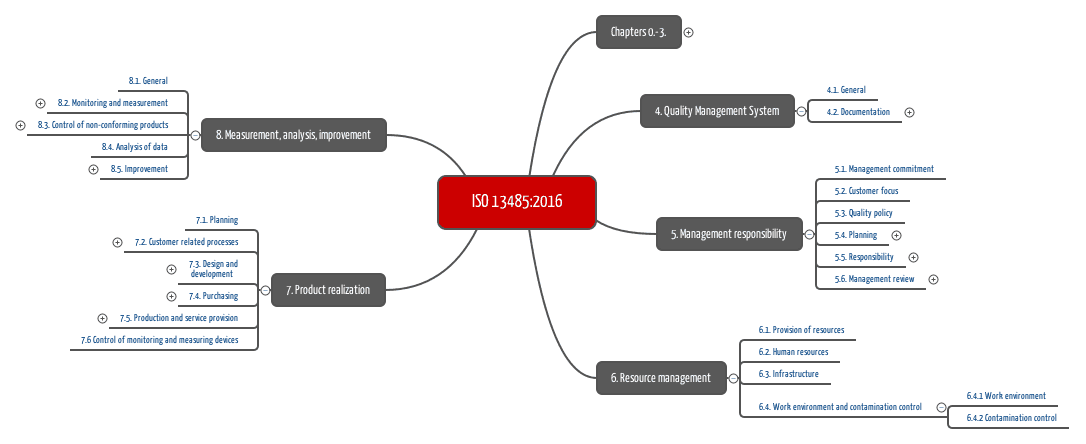
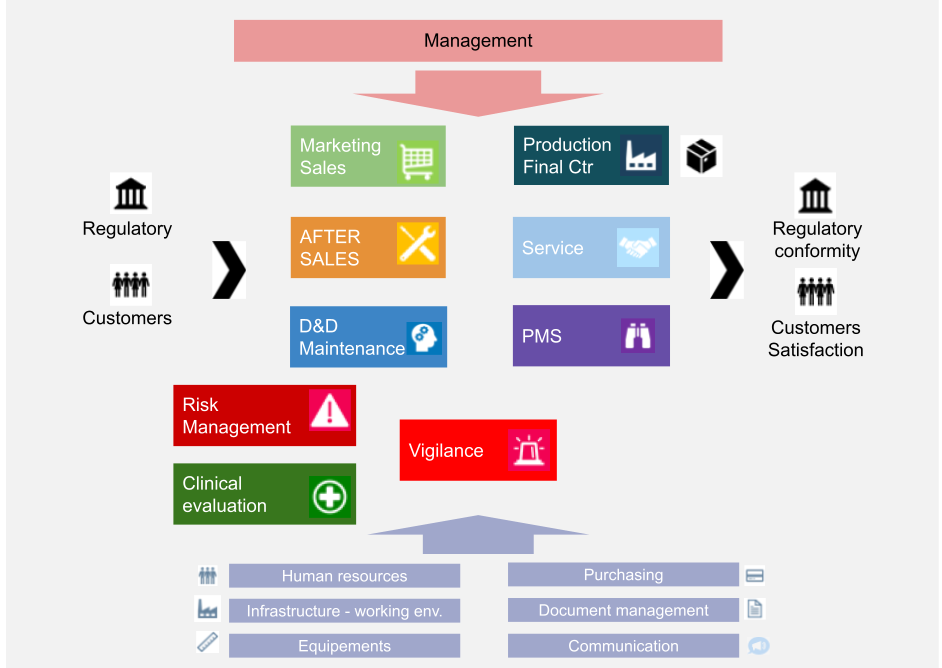
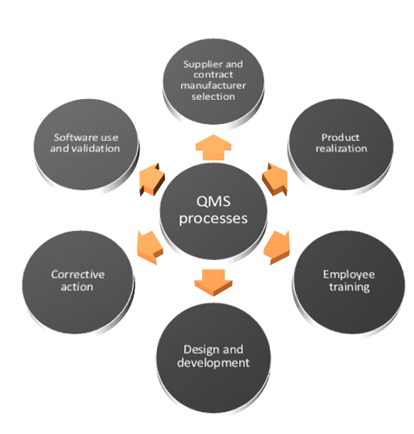
ISO 13485 Quality Management System for Medical Devices - o Singapore Consultancy Services for ISO, HACCP, CaseTrust, bizSAFE

ISO 13485 – Medical Device Quality Management System Requirements – ISO Templates and Documents Download
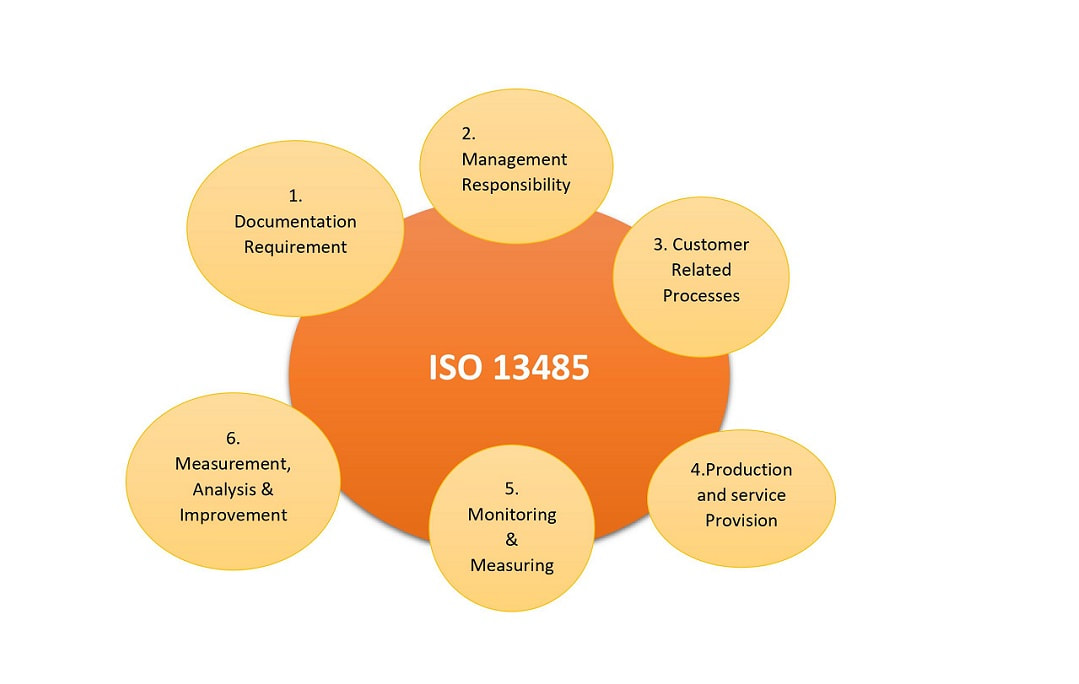
ISO 13485 Quality Management System for Medical Devices Certification Consulting - GlenView Group, Inc. - ISO Lead Auditor Certification Courses - Consulting & Training









.jpg)