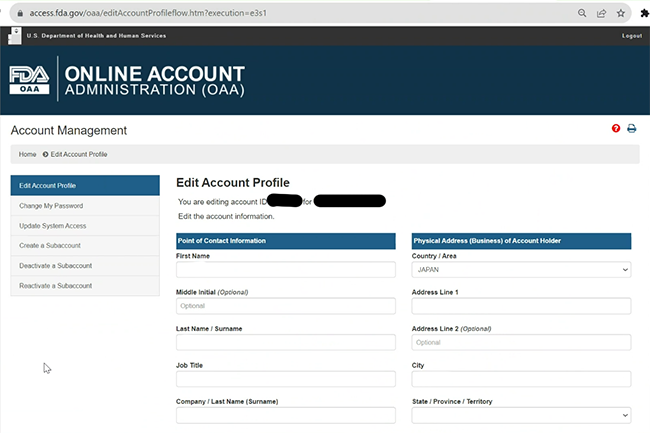
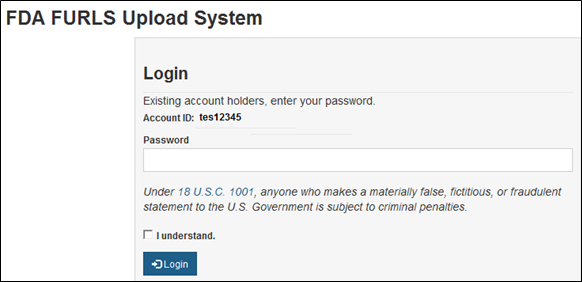
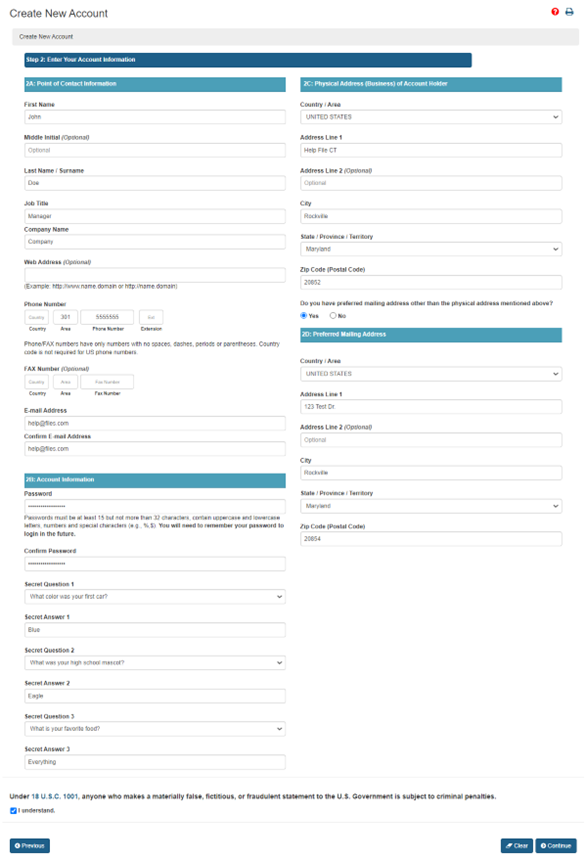
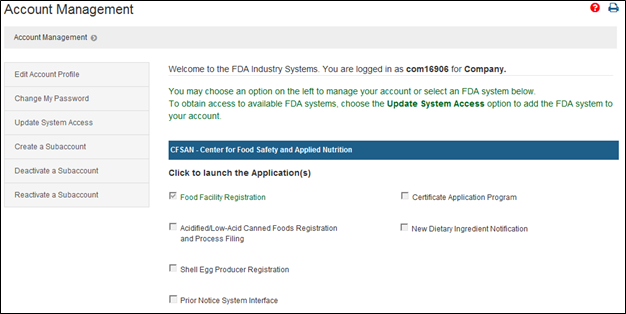
Veolia won the "first gold"! What made the first FDA certification in the China's plastic recycling industry so special? | Veolia China
28 avril 2023 : Microbiome Post - MaaT Pharma Announces U.S. FDA Lifts Clinical Hold on Phase 3 Investigational New Drug Application for MaaT013 in Patients with Acute Graft-versus-Host Disease (English only) - MaaT Pharma

The FDA and Worldwide Quality System Requirements Guidebook for Medical Devices: 9780873893770: Medicine & Health Science Books @ Amazon.com

FDA Drug Information on X: "FDA issued a final guidance that provides industry, investigators and others recommendations on the use of digital health technologies (DHTs) to acquire data remotely from participants in

Smoore Tech Invited to Address FDA Industry Meeting - Smoore International (06969) We are the world's leading atomization technology solution provider

Hairise FDA Approved Industry Modular Belt Conveyor System - China Chain Conveyor, Spiral Conveyor | Made-in-China.com