CTIS – M07 How to create a CT: Clinical Trial centric approach vs organisation centric approach - YouTube

EMA Webinar for SMEs and Academia on the Clinical Trials Regulation and the Clinical Trials Information System | ERICA

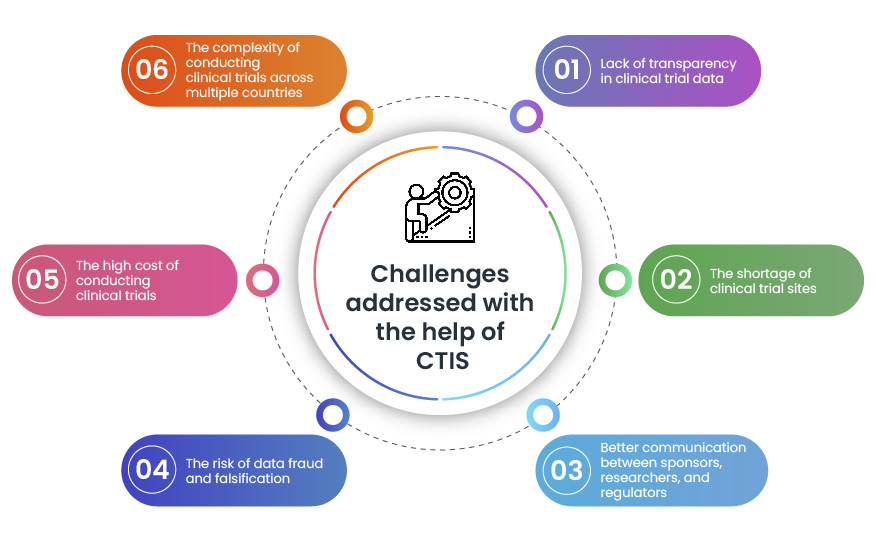
Managing the New EU Clinical Trials Regulation 536/2014 – Guidance for Navigating the Clinical Trial Information System (CTIS)